CAR-T Cell Treatment in Turkey
Car t cell treatment in Turkey ensures the highest standards of care, offering a comprehensive and safe solution for your health needs. DGS Healthcare helps you find the best CAR-T cell treatment in Turkey at affordable prices and adopts a 360-degree service approach in all areas of health through affiliated hospitals.
About CAR-T Cell Treatment in Turkey
CAR-T cell treatment is a personalized form of cell therapy that reprograms a patient’s own T cells to recognize and destroy specific cancer cells. In Turkey, specialist doctors carry out CAR-T therapy using established clinical protocols and modern manufacturing facilities or through accredited partners.
How it works (brief): T cells are collected from the patient’s blood (apheresis), sent to a lab where they are genetically modified to express a chimeric antigen receptor (CAR), expanded, then returned and infused back into the patient to target cancer cells.
CAR-T cell therapy has shown high response rates for certain blood cancers and is increasingly used in specialist hospitals in major Turkish cities. Evidence and approvals vary by indication and country; for up-to-date guidance on approved uses and safety, reputable sources include national regulators and major oncology guidelines (for example, FDA, EMA, and NCCN).
Typical process steps in Turkey: apheresis to collect T cells; laboratory genetic engineering to produce CAR-T cells; quality testing and expansion; lymphodepleting chemotherapy; and infusion of the manufactured CAR-T product. Each step follows strict quality and safety checks by doctors and manufacturing partners.
Approved indications differ between countries and evolve with new data. In many centers worldwide, CAR-T is approved for certain relapsed or refractory lymphomas and leukemias; other cancer types are being evaluated in clinical trials. Turkish specialists will assess eligibility, review medical history and prior treatments, and advise whether a patient is suitable for standard CAR-T therapy or a clinical trial.
Because CAR-T can cause significant side effects, patients are closely monitored before, during, and after treatment by multidisciplinary teams. Children with leukemia who may be candidates are typically referred by their treating physician to pediatric stem cell or CAR-T centers in Turkey for specialist review; DGS Healthcare can help arrange referrals and consultations with qualified doctors and hospitals in cities such as Istanbul, Ankara, and Antalya.
What Is CAR-T Cell Treatment in Turkey?
CAR-T is a personalized cell therapy that uses a patient’s own T cells (a type of white blood cell) engineered to recognize and kill cancer cells. In Turkey, CAR-T treatment is offered by specialist teams in major hospitals and centers that coordinate the collection, manufacturing, and clinical care required for this complex procedure.
Key points (simple):
- Collection: T cells are collected from the patient’s blood by apheresis.
- Manufacture: In a controlled lab the T cells are genetically modified to express a chimeric antigen receptor (CAR) and expanded into CAR-T cells.
- Infusion: After quality testing and a short preparatory chemotherapy (lymphodepletion), the CAR-T cells are infused back into the patient to target cancer cells.
Because CARs are designed to bind specific antigens on cancer cells, each CAR-T product targets particular cancers. For example, many approved CAR‑T therapies target CD19, a protein common on B‑cell leukemias and lymphomas. If a tumor does not express the target antigen, that CAR-T product will not be effective.
Who may be considered for CAR‑T?
- Patients with relapsed or refractory blood cancers (example: certain leukemias and large B‑cell lymphomas) after standard therapies.
- Children and young adults with relapsed acute lymphoblastic leukemia (eligibility varies by protocol and age limits).
- Patients who have relapsed after a bone marrow transplant or who are unsuitable for transplant for medical reasons.
Eligibility is determined case-by-case by Turkish specialist doctors after reviewing medical history, prior treatments, and necessary tests. For authoritative lists of approved indications and guidance on safety monitoring, clinicians commonly refer to regulatory bodies and oncology guidelines (for example, FDA, EMA, and national hematology societies).
Because CAR‑T can produce serious side effects (for example, cytokine release syndrome and neurotoxicity), patients are monitored closely in hospital or in specialized outpatient programs after infusion. Turkish centers offer multidisciplinary teams (doctors, nurses, intensive care support) to manage side effects and follow patients through recovery.
If you would like a preliminary eligibility check or help arranging a consultation with specialist doctors and accredited hospitals in Turkey, DGS Healthcare can assist with referrals, documentation review, and next steps.
How Is CAR-T Cell Treatment Performed in Turkey?
The CAR‑T treatment pathway in Turkey follows internationally accepted steps: referral and eligibility review, T‑cell collection (apheresis), laboratory manufacture of CAR‑T cells, short preparative chemotherapy (lymphodepletion), and infusion followed by close monitoring. Specialist doctors and multidisciplinary teams coordinate each stage to meet quality and safety standards.
Step 1 — Referral and Eligibility
- Referral: your treating doctor refers you or you contact a Turkish centre (DGS Healthcare can assist referrals and document transfer).
- Assessment: specialist doctors review your medical history, prior treatments, blood tests and scans to decide if CAR‑T is appropriate.
- Timing: because prior therapies can affect T‑cell quality, clinicians may pause some treatments to collect healthier cells when safe to do so.
Step 2 — Collection (Apheresis)
Apheresis is performed in a clinic or hospital: blood is circulated through a device that separates T cells and returns the remainder to you. The collected cells are frozen or shipped under controlled conditions to a manufacturing facility for genetic modification.
Step 3 — Manufacturing (3–6 weeks typical)
In the lab, T cells are activated, transduced (often using a viral vector) to add the CAR gene, expanded to therapeutic doses, tested for quality and safety, then cryopreserved. Manufacturing timelines vary by product but commonly range from about 3 to 6 weeks; your treating team will provide a schedule specific to your case.
Step 4 — Preparation and Infusion
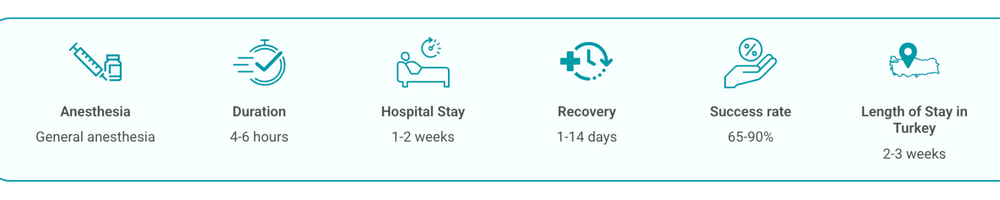
Before infusion you typically receive short-course lymphodepleting chemotherapy (commonly 2–3 days) to improve CAR‑T cell expansion. The cryopreserved CAR‑T product is thawed and infused intravenously, similar to a blood transfusion. After infusion the CAR‑T cells circulate, recognize target antigen‑positive cancer cells, become activated, multiply and exert anti‑tumor effects.
Early Recovery and Monitoring
Patients may be observed as inpatients for 1–2 weeks or more depending on how they respond. Alternatively, some centers manage selected patients as outpatients with daily checks for the first 2 weeks. Monitoring focuses on early complications such as cytokine release syndrome (CRS) and immune effector cell‑associated neurotoxicity (ICANS); intensive care support is available if needed.
Long‑Term Follow‑Up
Long‑term recovery varies; typical follow‑up visits occur around 30 days, 100 days and one year post‑infusion and include physical exams, blood tests, imaging and (when relevant) bone marrow evaluation. The CAR‑T team and your primary specialist coordinate ongoing care and surveillance.
Safety and Patient Selection
Because CAR‑T can cause serious side effects, careful patient selection and close post‑infusion monitoring are essential. Patients with significant comorbidities (severe heart, lung, liver, or kidney disease), active infection, or uncontrolled neurological conditions may not be eligible. Older patients (for example, 65+) require individualized risk assessment because of higher risk of some toxicities.
CAR‑T for Other Cancers & Clinical Trials
Research continues into CAR‑T for other blood cancers (multiple myeloma, other lymphomas) and solid tumours (brain, lung, prostate, melanoma, sarcoma, ovarian). Access for these indications is mainly via clinical trials; Turkish specialist centres participate in and can advise on suitable trials and eligibility.
If you want a practical next step, request a pre‑screening with DGS Healthcare to review medical records and determine whether a Turkish CAR‑T clinic or a clinical trial is the right option for your situation.

2025 Cost of CAR-T Cell Treatment in Turkey
The cost of CAR‑T cell treatment in Turkey is generally lower than in many developed countries, but final pricing depends on multiple factors. When DGS Healthcare coordinates care, the service can cover pre‑treatment evaluation, referrals, and help with logistics; however, itemized pricing is set by the treating hospital and the specific CAR‑T product or clinical trial.
What the quoted price typically covers
- Clinical assessment, eligibility workup and tests (blood work, imaging).
- Apheresis collection of T cells and transport to manufacturing facility.
- Manufacturing/manufacturer fees for the CAR‑T product (gene modification, expansion, QC).
- Hospital admission for lymphodepletion, infusion and immediate inpatient monitoring.
- Short‑term follow‑up care while in Turkey (nursing, routine outpatient checks included in package levels).
What is often excluded
- Extended ICU care for severe complications (billed separately if required).
- Long‑term monitoring once you return home (local care costs vary by country and insurer).
- Optional services (VIP accommodation upgrades, non‑standard transport) unless included in an all‑inclusive package.
Representative price ranges (published comparisons and market reports vary): the UK and USA prices for approved CAR‑T therapies are commonly reported in the high hundreds of thousands (£380,000–£470,000; $400,000–$450,000). In Turkey, typical total package ranges reported by providers are approximately $100,000–$300,000, depending on the product, hospital fees, and level of post‑op care included.
Prices may vary significantly by the CAR‑T product used, whether the cells are manufactured domestically or abroad, and whether the patient requires extended inpatient or ICU care. For example, complications that require prolonged hospital stay or additional treatments will increase overall costs.
Why Turkey can be more cost‑effective: favourable exchange rates for many international patients, a lower cost of living and medical overhead, and competitive pricing by hospitals that cater to international patients. Government incentives and market competition can also reduce out‑of‑pocket prices for foreign patients; still, quality and safety standards remain essential when comparing options.
If you need a precise, itemized quote, contact DGS Healthcare with recent medical records, pathology reports and imaging. We can coordinate an eligibility review with specialist doctors and obtain a tailored cost estimate that specifies what is included, expected timelines, and potential additional costs (for example, ICU care or extended follow‑up).
Note: the comparative prices cited above come from market reports and published figures; for clinical decision‑making and financial planning confirm current prices with your chosen hospital and the CAR‑T manufacturer, as costs and availability may change.
Why Choose Turkey for CAR-T Cell Treatment?
Turkey is an increasingly popular destination for international patients seeking advanced CAR‑T cell therapy. Leading hospitals and specialist clinics in Istanbul, Ankara, Antalya and other major cities offer multidisciplinary care teams, modern manufacturing partners and experience coordinating complex cell therapy procedures for patients from many countries.
Quality hospitals and specialist teams
- Many Turkish hospitals hold international accreditations (for example, JCI) and have dedicated units for cellular therapies and hematology-oncology.
- Care is provided by multidisciplinary teams (specialist doctors, nurses, ICU support and lab/ manufacturing partners) experienced in managing CAR‑T patients and treatment-related side effects.
Cost and value
Turkey often provides competitive pricing for CAR‑T cell therapy compared with Europe and North America due to favorable exchange rates for many foreign patients, lower local overheads and active medical tourism markets. That said, price should be considered alongside hospital quality, outcomes, and the inclusion of services (manufacturing, hospital stay, ICU access) when evaluating options.
Safety and outcomes
Leading Turkish centres follow international safety protocols for CAR‑T, including monitoring and management of cytokine release syndrome (CRS) and neurotoxicity (ICANS). When comparing clinics, patients should look for transparent outcome reporting, clinical experience (number of CAR‑T procedures performed), and published data where available.
Practical advantages for international patients
- Central flight hubs and direct connections make travel to major Turkish cities straightforward for many patients.
- Hospitals and medical facilitators often provide help with visas, local transport and accommodation; all‑inclusive packages can bundle medical and non‑medical services for convenience.
Choosing a hospital or doctor — verification checklist
- Check accreditation (JCI or national health authority) and whether the centre has a dedicated cell therapy unit.
- Ask about the centre’s CAR‑T experience (number of cases), published outcomes and the team’s ICU support capabilities.
- Request an itemized cost estimate that clarifies what is included (manufacturing, hospital days, ICU, follow‑up) and what may be extra.
While some pages list specific hospitals and doctors, patients should prioritise objective criteria (accreditation, experience, outcome data). DGS Healthcare can help by connecting patients with accredited clinics and by arranging a free eligibility assessment and itemized quote from specialist teams.
If you would like help verifying hospital credentials, reviewing published outcomes, or obtaining an itemized treatment and travel package that balances price and quality, contact DGS Healthcare for a no‑obligation consultation.

