NK-92 Cell Therapy in Turkey
Access advanced NK-92 cell therapy in Turkey — a cutting-edge cell therapy option available through specialist centres and coordinated by DGS Healthcare. We work with experienced clinical teams to help you explore treatment options, organise consultations and understand the pathway from assessment to follow-up.
NK 92 Cell Therapy Turkey
NK-92 cell therapy in Turkey is an emergent form of immunotherapy that offers an off‑the‑shelf cell therapy alternative for some cancer patients. The NK‑92 line is an IL‑2‑dependent natural killer (NK) cell line originally derived from a lymphoma patient and expanded in GMP‑compliant banks so clinicians can access consistent doses without harvesting cells from each individual.
Functionally, NK‑92 cells act like native NK cells: they detect stressed or abnormal tumour cells and release cytotoxic proteins (perforin and granzyme) to eliminate them. In clinical practice the cells are typically irradiated prior to infusion to prevent long‑term engraftment, which is one reason they have a low risk of graft‑versus‑host disease (GvHD) compared with donor lymphocyte approaches. As an experimental cancer treatment, NK‑92 therapy aims to provide a potent anti‑tumour immune response while maintaining a favourable safety profile.
If you are evaluating NK‑92 cell therapy in Turkey, DGS Healthcare can help arrange consultations with specialist teams, explain the likely pathway for assessment and treatment, and support practical steps such as eligibility checks and travel planning for this medical treatment.
NK 92 Cell in Turkey
NK‑92 cell therapy in Turkey is a form of adoptive immunotherapy in which patients receive infusions of a standardised NK cell line. Because NK‑92 is produced from a master cell bank under GMP conditions, clinics can deliver consistent off‑the‑shelf doses rather than collecting and manufacturing cells from each individual patient. The approach can be used as a stand‑alone cell therapy or as a platform for engineered products.
In research settings, NK‑92 cells are often modified with chimeric antigen receptors (CARs) to produce CAR‑NK products that recognise specific tumour markers. CAR‑NK‑92 allows targeted killing — for example, CARs against CD19 or CD20 are designed for B‑cell leukaemias and lymphomas, CD38 for myeloma, and HER2 for some breast and epithelial cancers. For lay readers: CAR‑NK simply means the NK cells have been given a synthetic receptor so they bind and kill cancer cells that display a chosen marker.
Preclinical studies show strong cytotoxicity of NK‑92 cells across multiple tumour types, and animal models typically report no durable engraftment after cells are irradiated pre‑infusion — a standard safety step that reduces the risk of long‑term proliferation and helps limit graft‑versus‑host disease (GvHD) risk. These features support the use of NK‑92 as a reproducible platform for both haemato‑oncologic and solid‑tumour applications, although most indications remain investigational and depend on ongoing clinical trials.
In Turkey, specialised centres and the doctors who run them can offer access to NK‑92 programmes — often as part of clinical studies or regulated experimental protocols. DGS Healthcare can connect you with experienced clinical teams, explain the available options and help coordinate assessments, so you can understand whether NK‑92 cell therapy is a suitable route in your individual case.
Why Consider NK‑92 Therapy?
NK‑92 cell therapy in Turkey offers several practical advantages compared with bespoke, patient‑derived cell products. Below are the key strengths that have made this approach attractive within clinical research and specialised treatment programmes.
“Off‑the‑shelf” availability: Doses are produced from a frozen master cell bank and expanded under GMP, removing the need for individual cell harvests. This shortens lead times and reduces variability versus autologous manufacturing.
No or low GvHD risk: Because NK‑92 products are irradiated before infusion and do not engraft long‑term, the risk of graft‑versus‑host disease is markedly lower than with some donor T‑cell therapies. This characteristic makes the therapy suitable for recipients without HLA matching in many trial contexts.
Consistency and potency: Each dose originates from a standardised cell line, which improves batch‑to‑batch consistency and predictability of activity. The innate cytotoxic mechanisms of NK cells can be further enhanced by genetic engineering (for example, CAR constructs) to increase tumour specificity.
Flexibility: Variants such as NK‑92MI (which expresses IL‑2) have been developed to reduce or remove the need for high‑dose systemic IL‑2, addressing a common toxicity concern. This engineering improves tolerability for some patients in clinical protocols.
Safety profile: Early clinical studies and case reports indicate that NK‑92 infusions are generally well tolerated; reported side effects tend to be mild and transient (eg, fever, fatigue). Severe cytokine release syndromes that can occur with some CAR‑T therapies have been uncommon in NK‑92 studies to date, but ongoing trials are continuing to define the safety profile across larger patient groups.
Overall, NK‑92 cell therapy in Turkey may offer a safer, more accessible cell therapy platform for selected patients within clinical or regulated experimental programmes. If you would like to discuss whether this cell therapy option is appropriate, ask your specialist or contact DGS Healthcare for an eligibility consultation and further information about potential benefits and risks.
Types of Cancer Treated with NK‑92 Cell Therapy
NK‑92 cell therapy in Turkey is being investigated across a range of malignancies. The therapy is particularly suited to tumours that display low MHC or high stress‑ligand expression and is being studied as an adjunct or alternative to established cancer treatments. Most indications remain experimental and are accessed via clinical trials or regulated programmes.
Haematologic malignancies: Early trials have reported activity of NK‑92 products in relapsed or refractory lymphomas and myeloma. A small Phase I study demonstrated complete remissions in some heavily pretreated patients, and ongoing studies are evaluating CAR‑NK‑92 constructs for acute myeloid leukaemia (AML) and acute lymphoblastic leukaemia (ALL). These programmes illustrate how cell therapy can be applied where standard options have been exhausted.
Lung cancer and metastases: Preclinical studies and early human work suggest NK‑92 infusions can mediate on‑target killing of lung tumours and metastases with limited off‑target effects. Trials are underway in several centres to better define efficacy and optimal delivery methods for thoracic disease.
Solid tumours: NK‑92 has been used experimentally in refractory solid tumours. A published case report from Turkey described tumour shrinkage in a child with relapsed Ewing sarcoma following intratumoural NK‑92 therapy, without significant toxicity. Research continues into CAR‑armed NK‑92 approaches for breast cancer, glioblastoma and pancreatic cancer, aiming to overcome barriers that limit immune cell infiltration of solid masses.
Engineered targets: CAR‑NK‑92 constructs expand the range of tumour markers that can be targeted. Examples under study include CD19/CD22 or CD20 for B‑cell disorders, CD38 for myeloma and HER2 for HER2‑positive breast and epithelial cancers. These engineered therapies combine the NK‑92 platform’s consistency with antigen‑specific targeting to improve therapeutic precision.
Summary: NK‑92 cell therapy in Turkey offers a versatile platform that is being trialled against haematologic cancers, lung tumours and a range of solid tumours, often within CAR‑NK research programmes. If you are considering participation in a study or seeking treatment options, DGS Healthcare can help identify relevant trials, provide information on trial endpoints (response rates and safety) and support your application to the appropriate centres.
Who Is a Candidate for NK‑92 Cell Therapy?
NK‑92 cell therapy in Turkey is typically offered within clinical trials or under regulated experimental programmes. Eligibility varies by protocol, but the following categories represent common inclusion considerations used by doctors evaluating candidates for this cell therapy:
Relapsed or refractory disease: Patients whose cancer has progressed despite standard options (chemotherapy, radiation therapy, surgery or approved immunotherapies) are often considered for NK‑92 trials, especially when other treatments are exhausted.
Good performance status: Most trials require patients to have reasonable fitness (for example, ECOG 0–2) so they can tolerate infusions and follow‑up assessments. Age criteria differ by study; some recruit adults only while others include paediatric patients under specific protocols.
Adequate organ function: Eligibility usually requires acceptable laboratory values (examples from published protocols include creatinine <2× normal, AST/ALT <5× normal, normal bilirubin) and sufficient cardiac function (often LVEF ≥45%). Exact thresholds vary by trial, so clinicians will review your blood tests and imaging to confirm suitability.
Measurable disease: Trials generally require evidence of active disease (tumour measurable on imaging or detectable blasts in blood/marrow) so investigators can evaluate response to the therapy.
Informed consent and regulatory approval: Participation requires full informed consent; many programmes are not approved by the FDA (US) and are run under local regulatory oversight in Turkey. Ethical approval and trial enrolment are mandatory for experimental protocols.
In practice, eligibility is assessed case‑by‑case. If you think you or a loved one might qualify, specialist doctors in Turkey can review prior treatments and recent blood tests to determine suitability. DGS Healthcare can assist by arranging an initial eligibility check and providing guidance upon request.
Before NK‑92 Cell Therapy in Turkey
Prior to treatment, patients typically undergo a preparatory assessment that includes baseline blood tests (CBC, kidney and liver panels, infection screening), cardiac evaluation (for example, echocardiography), and imaging as appropriate. Women of childbearing potential are usually required to have a negative pregnancy test and agree to contraception during the study period. Recent chemotherapy or radiation therapy is commonly paused for a short window (varies by protocol) to reduce overlapping toxicities.
Patients may be asked to stop certain immunosuppressive or myelosuppressive medicines before enrolment; clinicians will advise on timing. Vaccinations such as the seasonal influenza vaccine may be recommended to optimise overall health prior to cell therapy. Your treating team will provide a detailed checklist of required blood tests and documents — DGS Healthcare can help coordinate these steps and provide a free initial consultation to explain what is required.
How is NK‑92 Cell Therapy Performed in Turkey?
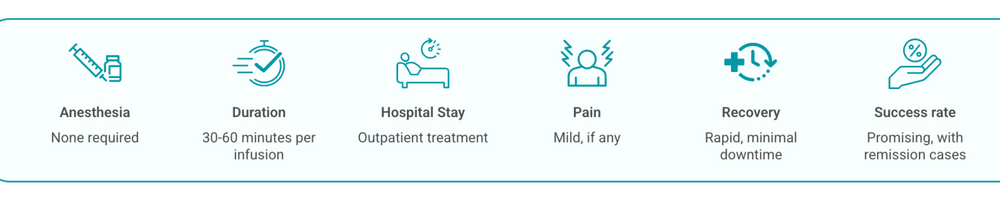
NK‑92 cell therapy programmes in Turkey follow a stepwise pathway to ensure patient safety and to monitor response. The clinical team will assess suitability, perform baseline tests and plan the logistics of each infusion. Below is a concise, patient‑facing summary of the typical process used in centres that perform these experimental cell therapy treatments.
Pre‑treatment assessment
Before any infusion, clinicians conduct a comprehensive evaluation including blood tests (CBC, kidney and liver panels, infection screening), cardiac assessment (for example, echocardiography) and imaging to confirm measurable disease. Recent chemotherapy or radiation therapy is usually paused per protocol to minimise overlapping toxicities; the exact pause period varies by trial. Women of childbearing potential must have a negative pregnancy test and agree to contraception during the treatment period.
Infusion day — procedure
On the day of infusion, the prepared NK‑92 cells (manufactured in a sterile GMP laboratory and typically irradiated to prevent proliferation) are delivered intravenously. Clinicians commonly administer standard pre‑medications such as paracetamol (acetaminophen) and antihistamines to reduce immediate reactions. The infusion itself usually lasts 30–60 minutes; typical dose ranges used in early clinical work have been reported in the order of 1×10^9 to 5×10^9 cells per m2, but doses and schedules vary by protocol and indication.
During and immediately after infusion, the medical team monitors vital signs closely for any acute reactions. Mild side effects such as fever, chills or fatigue are common and are usually managed with supportive care. Severe cytokine release syndromes have been uncommon in published NK‑92 studies to date, but ongoing trials continue to define safety in larger cohorts.
Treatment schedule and follow‑up
If tolerated, infusions are usually given in cycles. Typical early‑phase schedules have repeated doses every 3–4 weeks, with total planned cycles ranging from 2 to 6 depending on the protocol and the patient’s clinical response. Between cycles, clinicians assess safety with blood tests and monitor tumour response using imaging studies. Your treating doctor will tailor the schedule according to the trial design and your individual condition.
After NK‑92 Cell Therapy in Turkey
Post‑infusion care is generally straightforward. Most patients experience only short‑lived flu‑like symptoms (fever, fatigue, mild chills) that resolve with simple measures such as paracetamol and rest. There are no routine long‑term infection precautions because NK‑92 therapy does not usually cause prolonged immune suppression. Still, regular follow‑up is essential: clinicians perform periodic blood tests to monitor organ function and use imaging to evaluate tumour response. If symptoms persist beyond 24–48 hours or if new concerns arise, patients should contact their medical team promptly.
Statistics of NK‑92 Cell Therapy in Turkey
NK‑92 programmes remain investigational worldwide. Early Phase I studies (for example, a Toronto study in relapsed blood cancers) administered high‑dose irradiated NK‑92 infusions and reported objective responses in some heavily pretreated patients; however, sample sizes were small and further trials are required to establish efficacy. Case reports — including a published Turkish paediatric case of intratumoural NK‑92 in relapsed Ewing sarcoma — describe tumour shrinkage without significant toxicity. Across all studies to date, several dozen to around a hundred patients have received NK‑92 or CAR‑NK‑92 products in clinical research, but numbers and outcomes evolve rapidly as new trials open.
Future Applications of NK‑92 Cell Therapy
Research is extending NK‑92 applications to challenging solid tumours (for example, glioblastoma and pancreatic cancer) and enhancing the platform with CAR engineering, combination regimens with chemotherapy, radiotherapy or other immunotherapies, and strategies to improve tumour infiltration. These future developments aim to increase response rates while maintaining a favourable safety profile; participation in such trials is currently the primary route for access to these novel medical treatments.
2025 Cost of NK‑92 Cell Therapy in Turkey
Turkey is a competitively priced destination for NK‑92 cell therapy and other advanced cell therapy options. The overall cost you will pay depends on the specific protocol, the number of infusion cycles, hospital stay, pre‑ and post‑treatment tests and any additional procedures. DGS Healthcare can provide a tailored cost estimate and help you understand what is — and is not — included in a package.
As of 2025, approximate comparative price ranges from different countries (indicative only) are shown below. These figures are illustrative — actual procedure cost will vary by centre, treatment complexity and included services.
Price of NK‑92 Cell Therapy in the UK
Indicative range: £80,000 – £150,000.
Price of NK‑92 Cell Therapy in the USA
Indicative range: $100,000 – $250,000.
Price of NK‑92 Cell Therapy in Turkey
Indicative range in Turkey: $30,000 – $100,000. This typically reflects procedure cost, hospital fees and basic follow‑up; exact inclusions vary by package. Contact us to get free, personalised pricing and a clear breakdown of what is covered.
Prices are indicative and may change depending on the treatment plan, the number of cycles, laboratory tests, imaging and any additional procedures. Contact us for an exact procedure cost and a free price breakdown.
Why Is NK‑92 Cell Therapy Cheaper in Turkey?
Several factors contribute to lower comparative prices for NK‑92 cell therapy in Turkey:
- Favourable exchange rates: For patients paying in euros, dollars or pounds, Turkey’s pricing can be cost‑competitive.
- Lower operating costs: The general cost of living and labour is lower than in many developed countries, reducing medical service fees.
- Medical tourism efficiencies and incentives: Clinics that work with international patients often offer bundled packages, and government incentives can further improve price competitiveness.
When evaluating offers, balance price with safety: look for hospitals with recognised accreditation (for example, JCI), experienced doctors and clear patient reviews. DGS Healthcare can provide verified reviews and help you compare packages upon request.
Every year many international patients choose Turkey for advanced cell therapy because specialist centres in Istanbul, Ankara and Antalya provide experienced clinical teams and comprehensive care. If you are considering travel for treatment, DGS Healthcare organises all‑inclusive packages on request — including hospital coordination, accommodation options, VIP transfers and aftercare planning — so you can focus on treatment and recovery.
What a Turkey package commonly includes
- Initial eligibility assessment and coordination with specialist doctors
- Hospital fees related to the NK‑92 procedure and a defined number of follow‑up visits
- Pre‑procedure blood tests and imaging (specified in the package)
- Hotel accommodation options and VIP transfers to/from the airport and clinic
- Assistance with travel logistics and local support during your stay
Not all packages include flights, visas or extended rehabilitation abroad — ask for a detailed quote so you can compare like‑for‑like. To get free information about the exact cost and what a bespoke package would cover, contact DGS Healthcare and request a personalised estimate.
Choosing a safe centre
Prioritise centres with clear clinical governance, experienced multidisciplinary teams and positive patient reviews. Ask for details such as hospital accreditation, names and CVs of the supervising doctors, and any published trial IDs or outcome data. DGS Healthcare can share verified reviews and typical timelines so you can select a provider with confidence.
If you would like help comparing prices, understanding procedure cost components, or receiving a free personalised quote and package outline, contact DGS Healthcare — packages can be arranged upon request to suit short or long stays and individual clinical needs.

